日本扁柏(Chamaecyparis obtusa)精油通过环氧化酶-2途径对大鼠的抗炎作用
 时间:2019-05-20 06:21:42
时间:2019-05-20 06:21:42  浏览量:5999 字号:
浏览量:5999 字号:


文章来源于国外网站,翻译为网站翻译,原文负载在下方
Authors:
Beum-Soo An Ji-Houn Kang Hyun Yang Eui-Man Jung Hong-Seok Kang In-Gyu Choi Mi-Jin Park
摘要
精油是含有来自植物的挥发性芳香化合物的浓缩疏水液体。在本研究中,提取了商业上用于肥皂,牙膏和化妆品的Chamaecyparis obtusa(C. obtusa)精油。从C. obtusa中提取的精油含有几种类型的萜烯,已经证明它们具有抗氧化和抗炎作用。在本研究中,我们在大鼠中通过脂多糖(LPS)诱导炎症后在体内和体外检查了C. obtusa精油的抗炎作用。虽然LPS通过在血液和外周血单核细胞(PMNCs)中产生前列腺素E2(PGE2)诱导炎症反应,但是当预先施用精油时这些水平降低。另外,通过测量炎症相关基因的mRNA表达来研究C. obtusa精油的抗炎作用的作用机制。LPS处理显着诱导大鼠转化生长因子α(TNFα)和环氧合酶-2(COX-2)的表达,而C. obtusa精油抑制这种作用。总之,我们的结果表明C. obtusa精油通过COX-2途径调节PGE2和TNFα基因表达的产生而发挥抗炎作用。这些发现表明C. obtusa精油可能构成消炎药的新来源。LPS处理显着诱导大鼠转化生长因子α(TNFα)和环氧合酶-2(COX-2)的表达,而C. obtusa精油抑制这种作用。总之,我们的结果表明C. obtusa精油通过COX-2途径调节PGE2和TNFα基因表达的产生而发挥抗炎作用。这些发现表明C. obtusa精油可能构成消炎药的新来源。LPS处理显着诱导大鼠转化生长因子α(TNFα)和环氧合酶-2(COX-2)的表达,而C. obtusa精油抑制这种作用。总之,我们的结果表明C. obtusa精油通过COX-2途径调节PGE2和TNFα基因表达的产生而发挥抗炎作用。这些发现表明C. obtusa精油可能构成消炎药的新来源。
介绍
炎症是由组织损伤引起的临床疼痛的最常见原因。疼痛的主要功能是保护机体免受潜在的组织损伤刺激。药物治疗炎症的目的是使疼痛敏感性正常化; 然而,目前可用的药物与严重的副作用和低效率相关。非甾体类抗炎药(NSAIDs)已被证明可有效治疗各种炎症疾病,与类固醇相比副作用更少(1))。NSAID通过与环加氧酶(COX)酶结合起作用以抑制花生四烯酸产生前列腺素。存在COX蛋白的两种同种型,COX-1和-2; COX-1在大多数器官和细胞中组成型表达,而COX-2通常不存在并且在炎性刺激(包括内毒素脂多糖(LPS))刺激后诱导。这表明,COX-2是一种特异性炎症同种型(1 - 3)。NSAIDs和COX-2选择性抑制剂是目前用于治疗几种炎性病症(4 - 6)。然而,COX-2的选择性抑制剂与心肌梗塞和中风的风险相关。常规NSAIDs和COX-2选择性抑制剂的由于它们的不良心血管和胃的副作用,最近戒断导致的替代抗炎剂(显影7 - 9)。因此,植物来源的天然药剂,例如精油,被认为是下一代抗炎药的有用来源。
精油是含有来自植物的挥发性芳香化合物的浓缩疏水液体。它们通过蒸馏,表达或溶剂提取物提取,并用于香水,化妆品,肥皂和其他产品中,用于向香料和家用产品添加香味以及用于调味食品和饮料。Chamaecyparis obtusa(C. obtusa)是一种在日本和韩国南部地区发现的热带树种,由于其结构特性和天然香味的优势,被用于建筑和家具。它的精油是从C. obtusa 树的修剪的叶子和树枝中提取的,并且已经在商业上用作肥皂,牙膏和化妆品作为功能性添加剂。该C.扁柏精油含有几种类型的萜烯,已被证明具有抗氧化和抗炎作用,包括桧烯,柠檬烯,乙酸龙脑酯,冰片,α-松油醇和elemol,而来自水果的精油含有月桂烯,γ-萜品烯,对甲基异丙基苯,冰片,α萜品醇,β石竹烯(10,11)。
在本研究中,在体内和体外研究了来自C. obtusa精油的抗炎特性,并确定了C. obtusa 作为新药物来源的能力。
期刊与论题
动物和治疗
雄性Sprague-Dawley(SD)大鼠(9周龄)获自Koatech Co.,Ltd。(Pyeongtaek-si,Korea)。在使用之前,将所有动物饲养在聚碳酸酯笼中并在环境控制室(23±2℃;相对湿度,50±10%;频繁通气;和12小时光照/黑暗循环)中适应环境(如前所述)(12)。本研究中使用的C. obtusa精油由Enbita Co.,Ltd。(Seoul,Korea)提供,并且 根据先前描述的方法(13)通过蒸汽蒸馏C. obtusa树的修剪枝和叶来生产。用C.obtusa处理大鼠精油调查其影响。向大鼠施用总共50ml自来水或精油溶液(25,50或100%),持续2周。对照组大鼠用盐水溶液(0.9%NaCl)处理,剩余的大鼠(处理组)用0,25,50或100%的C. obtusa溶液处理。存在或不存在LPS的精油(Sigma-Aldrich,St.Louis,MO,USA)。在处死前8小时,通过腹膜内(ip)注射给予LPS处理组(LPS阳性组)中的大鼠1μg/ kg LPS; 对照组LPS(LPS阴性组)的大鼠在LPS的同一时间点注射等体积的生理盐水。所有的实验程序都经过忠北大学(韩国清州)伦理委员会的批准。
外周血单核细胞(PMNC)分离
如先前所描述(利用双密度梯度离心法PMNCs从大鼠末梢血管分离14,15)。简言之,将肝素化血液样品覆盖在Histopaque-1077溶液(比重,1.077; Sigma-Aldrich)和Histopaque-1119溶液(比重,1.199; Sigma-Aldrich)上,以700×g离心40分钟并洗涤三次。用冷的磷酸盐缓冲盐水(PBS)。通过台盼蓝染料排除测定的PMNCs的存活率> 97%。将PMNCs重悬于补充有2mM L-谷氨酰胺,0.02mg / ml庆大霉素和5%热灭活的胎牛血清(FBS; Invitrogen,Grand Island,NY,USA)的RPMI-1640培养基(Sigma-Aldrich)中以供使用。在随后的实验中。
前列腺素E2(PGE2)ELISA
对于体内实验,在存在或不存在LPS的情况下,用各种C. obtusa精油溶液处理大鼠。处理后,从外周血管收集血清并进行PGE 2 ELISA测定。对于体外测定,用精油溶液处理大鼠2周。从大鼠外周血管收获PMNCs,并以0.5×10 5的密度接种在24孔板上。然后用10ng / ml LPS刺激PMNCs指定的时间,并收集上清液。对于PGE 2 ELISA测定,使用单克隆PGE 2分析收集的血清和上清液 ELISA试剂盒(Cayman Chemical,Ann Arbor,MI,USA)。
通过RT-PCR定量mRNA
使用TRIzol试剂(Invitrogen)提取总细胞RNA,并在260nm处测定RNA的浓度。如前所述进行RT-PCR(13)。简而言之,使用M-MLV逆转录酶(Invitrogen)和随机引物(9-mer; Takara Bio,Inc.,Shiga,Japan)将总RNA(1μg)逆转录成互补DNA(cDNA)。的cDNA(1微升)用于PCR在标准条件下(13,16); 在95℃变性30秒,在55℃退火30秒,在72℃延伸1分钟。使用的引物序列如下:COX-2有义,5'-TACCCGGACTGGATTCTACG-3'和反义,5'-AAGTTGGTGGGCTGTCAATC-3'; TNFα有义,5'-CTGAGCTCAAGCCCTGGTAT-3'和反义,5'-GGTCAGAGTAATGGGGGTCA-3'; 和细胞色素c氧化酶亚基1(1A)有义,5'-CCAGGGTTTGGAATTATTTC-3'和反义,5'-GAAGATAAACC CTAAGGCTC-3',其用作内部对照。在2%琼脂糖凝胶上分离PCR产物(10μl)并用溴化乙锭(EtBr)染色。使用Quantity One软件(Gel Doc EQ; Bio-Rad,Hercules,CA,USA)捕获并分析凝胶图像。
统计分析
使用Kruskal-Wallis检验通过非参数单向方差分析对数据进行分析,然后进行Dunnett检验以进行多重比较。数据值被转换为这些测试的等级。所有统计分析均使用SPSS for Windows(SPSS,Inc.,Chicago,IL,USA)进行。P <0.05被认为表示统计学上显着的差异
结论
C. obtusa精油对血清PGE2浓度的影响
研究了来自C. obtusa精油的血清PGE 2的调节。用不同浓度的精油处理大鼠2周,注射1μg/ kg LPS以在用精油处理后诱导炎症反应。
为了分析血清PGE水平2,具有用于PGE特异性抗体ELISA 2进行。用25mg / kg剂量的精油处理的动物中LPS诱导的血清PGE 2水平显着降低(图1)。其他浓度的 C. obtusa精油(50和100 mg / kg)也降低了血清PGE 2的水平; 然而,这些结果没有统计学意义。接下来,使用PMNCs评估精油的作用,以验证大鼠中血清PGE 2的增加。用不同浓度的精油处理2周后分离PMNCs,给予10ng / ml LPS 12(图2A)或24小时(图2B)。通过减少PMNCs中的PGE 2分泌,精油显示出对LPS诱导的炎症具有保护作用。在所有检测的剂量下用精油处理抑制12小时LPS诱导的PGE 2分泌水平,并且在50mg / kg的剂量下观察到最大抑制。LPS处理诱导的PGE 2产生抑制24小时仅在施用100mg / kg精油时才显示出显着性。
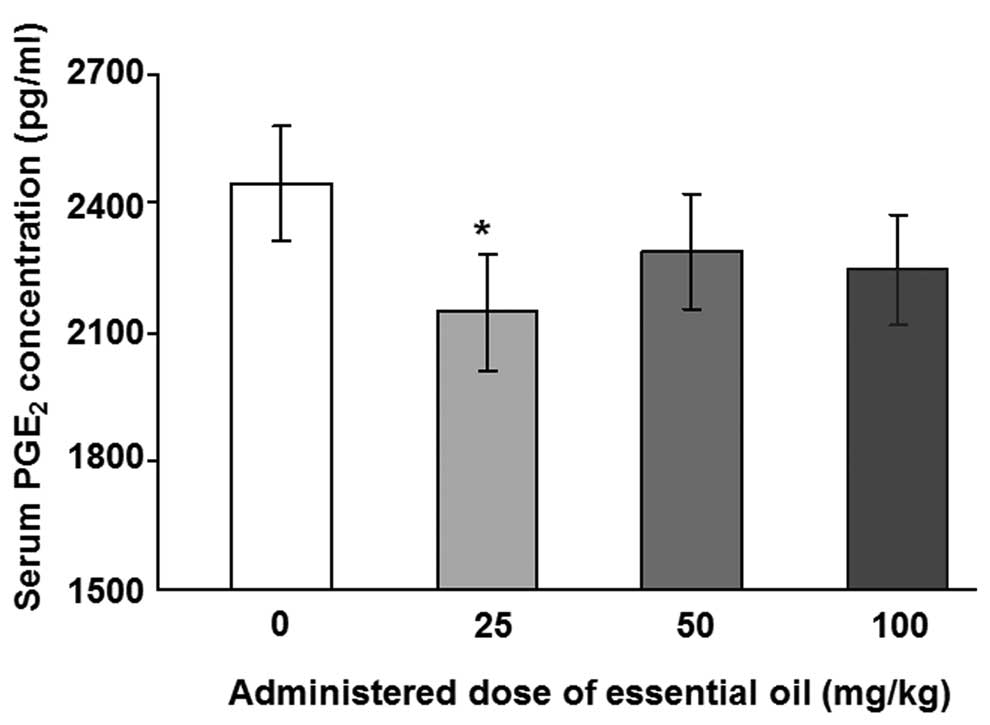 | 图1不同剂量的日本扁柏精油对脂多糖(LPS)诱导的大鼠血清中前列腺素E2(PGE2)浓度的影响。通过口服精油治疗大鼠2周,并在治疗的最后一天腹膜内注射1μg/ kg LPS。在LPS注射后8小时从大鼠血液样品中收集血清样品。用0.45μm孔径的膜过滤血清样品,并使用ELISA测定PGE2浓度。*与对照组相比,P = 0.036,通过单因素方差分析和随后的Dunnett检验(n = 4)确定。 |
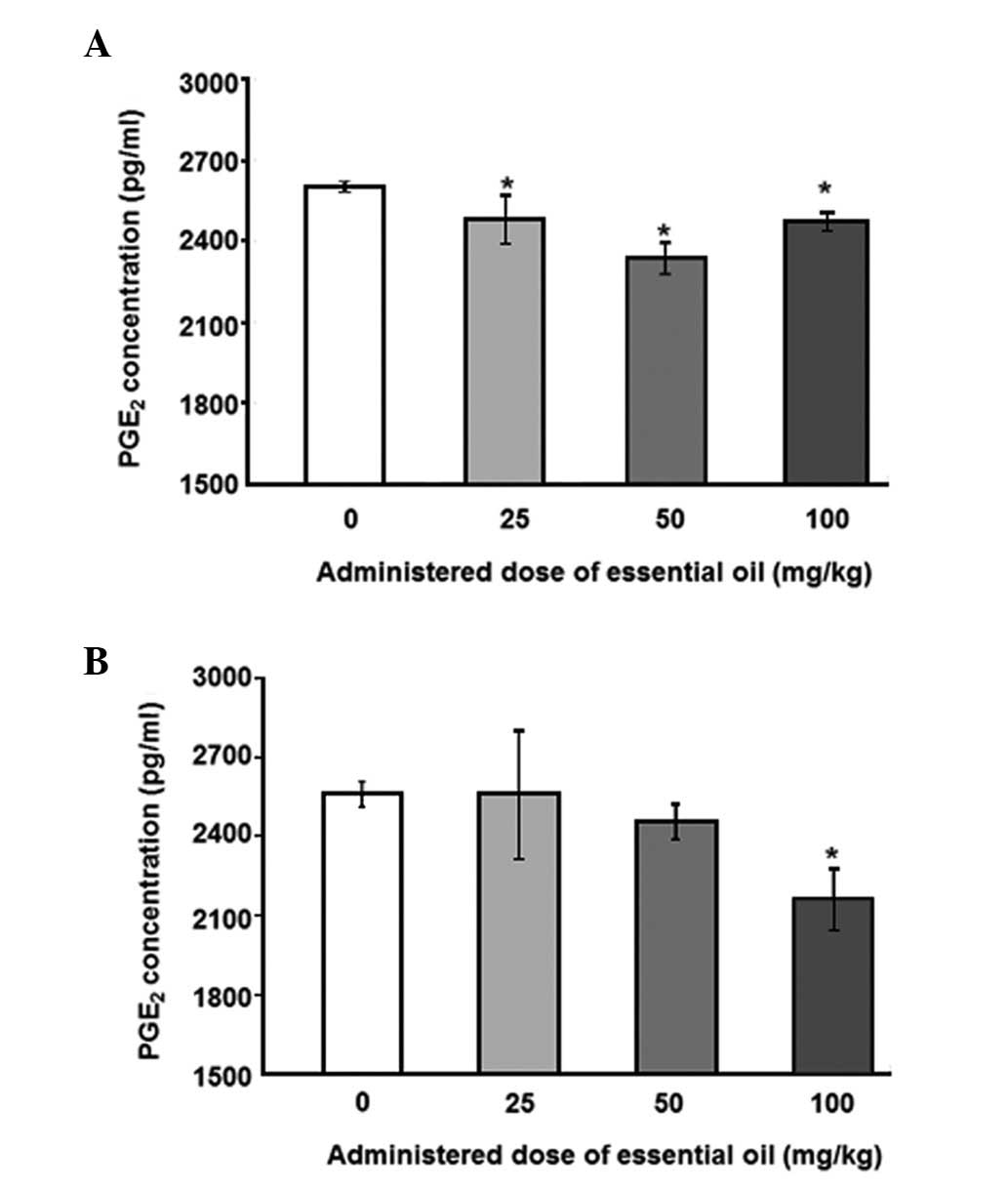 | 图2不同剂量的日本扁柏(Chamaecyparis obtusa)精油对脂多糖(LPS)诱导的外周血单核细胞(PMNCs)产生前列腺素E2(PGE2)的影响。从通过口服精油处理2周的大鼠获得的血液样品中分离PMNCs。对于(A)12或(B)24小时,通过10ng / ml LPS刺激分离的PMNCs,收获培养物上清液并用0.45μm孔径的膜过滤。使用ELISA测定培养上清液中的PGE 2浓度。*与对照组相比,P≤0.001,通过单因素方差分析,然后进行Dunnett检验(n = 4)。 |
C. obtusa精油对肺组织炎症相关基因TNFα和COX-2表达的影响
为了检查精油的抗炎作用可能的作用机制,我们分析了在存在或不存在LPS的情况下用精油处理后大鼠肺中炎症相关基因的表达。正如预期的那样,LPS增强了TNFα的表达(图3)。通过以剂量依赖性方式施用精油来降低肺中LPS诱导的TNFα表达,其中在100mg / kg的剂量下观察到最有效的作用(56%减少)。
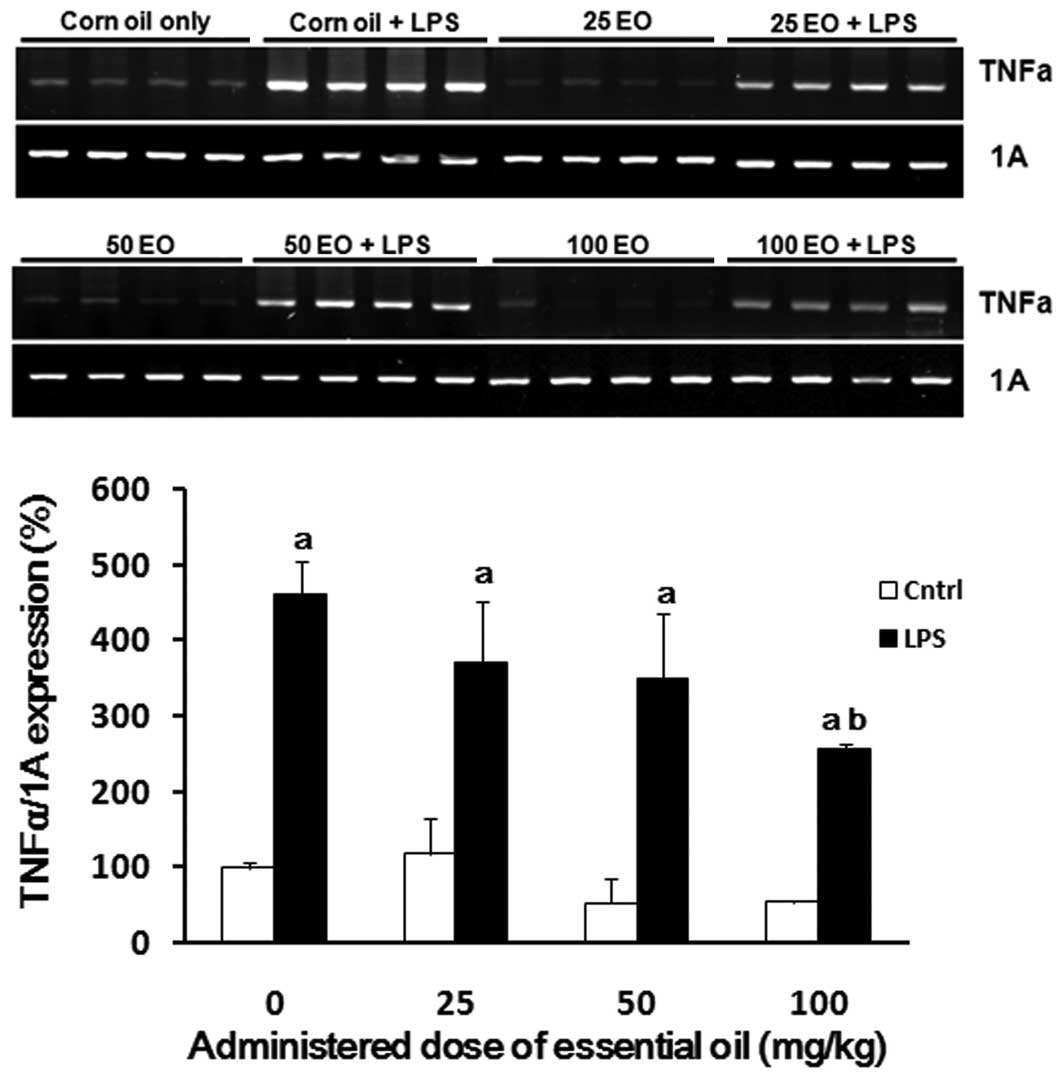 | 图3日本扁柏(Chamaecyparis obtusa)精油(EO)对脂多糖(LPS)诱导的肿瘤坏死因子α(TNFα)mRNA表达的影响。通过口服各种剂量的精油治疗大鼠2周,并在治疗的最后一天腹膜内注射1μg/ kg LPS。在LPS注射后8小时从大鼠的肺中提取mRNA样品,并如材料和方法中所述通过RT-PCR用TNFα引物进行分析。aP <0.05与LPS阴性组相比; 与LPS阳性对照组相比,bP <0.05。 |
还评估了COX-2 mRNA表达以确定精油发挥其抗炎作用的机制(图4)。与对照组相比,通过LPS处理(高达5倍)增加COX-2的表达(图4)。通过预先施用精油来抑制LPS处理对COX-2表达的诱导; 这种抑制作用在50mg / kg的剂量下最显着。
 | 图4日本扁柏(Chamaecyparis obtusa)精油(EO)对脂多糖(LPS)诱导的环氧合酶-2(COX-2)mRNA表达的影响。通过口服各种剂量的精油治疗大鼠2周,并在治疗的最后一天腹膜内注射1μg/ kg LPS。在LPS注射后8小时从大鼠的肺中提取mRNA样品,并通过RT-PCR分析COX-2表达。aP <0.05与LPS阴性组相比; 与LPS阳性对照组相比,bP <0.05。 |
讨论
自古以来,植物已被用于其药用特性,其部分归因于精油(17)。精油是天然,复杂和多组分油,主要由萜烯和其他成分组成(18)。精油广泛用于预防和治疗人类疾病,包括癌症和心血管疾病。此外,他们之前已经报道了它们作为抗细菌剂,抗病毒剂,抗氧化剂和抗糖尿病药的生物活性(18)。最近的研究已经从一些植物(证明精油的抗发炎的特性,19 - 21)。在本研究中,我们证明了来自C. obtusa的精油 对LPS诱导的大鼠炎症具有抗炎作用。 当用精油处理大鼠时,PGE 2的血清浓度降低。此外,从血液PMNCs 合成PGE 2显示出与血清相同的结果。这些结果表明PGE 2在LPS刺激后由血液单核细胞合成,并且这种炎症过程由C. obtusa精油改善。
PGE 2在体内产生并通过刺激COX途径与LPS处理一起累积。PGE 2的定量分析代表体内和体外的炎症活性(22)。在本研究中,LPS诱导的PGE 2生产是在血清和大鼠的PMNCs通过与处理降低C.扁柏精油。先前已报道过类似的结果; 通过抑制PGE 2和一氧化氮的产生,已显示Hyptis pectinata精油具有抗炎作用(23)。柳杉(Cryptomeria japonica)精油已被证明可抑制LPS诱导的巨噬细胞中PGE 2和细胞因子的产生,包括白细胞介素(IL)-1β和IL-6(24); 此外,分析了日本柳杉精油的化学成分,其主要成分为elemol和sabinene。Elemol和桧也已知的萜类 C.扁柏精油(10,11)。
在本研究中,我们研究了 C. obtusa精油对LPS处理后TNFαmRNA表达调节的影响。正如所料,LPS处理后大鼠肺组织中TNFα的表达增加,这意味着LPS诱导免疫应答。此外,用C. obtusa精油处理消除了TNFα的诱导表达。验证C. obtusa的抗炎作用的作用机制 精油,我们还检查了COX-2表达水平的调节。病原体相关的免疫应答是抗原性的,并且能够激活单核细胞或巨噬细胞,以合成各种炎性细胞因子,包括IL-1,IL-6和TNF,已经报道通过COX表达(被介导的24 - 26)。用LPS处理后COX-2的表达增加,并且这种上调被C. obtusa精油以剂量依赖性方式抑制。这些结果表明,这种精油的抗炎作用是通过COX-2介导的PGE2和TNFα信号通路的调节而发生的。
先前已经证实了精油通过COX-2信号通路的抗炎作用; 鱼腥草挥发油通过抑制LPS诱导的PGE 2产生而具有抗炎作用(9)。在这项研究中,精油的抗炎作用是通过抑制COX-2基因表达介导的,而COX-1,非炎症特异性同种型,不参与。Katsuawa 等(27)也证明了柠檬草精油可抑制人类巨噬细胞样U937细胞中的COX-2启动子活性。
总之,我们的研究结果表明,C。obtusa精油通过调节血液中PGE 2的产生和大鼠中TNFα的基因表达来发挥其抗炎作用。C. obtusa精油的抗炎作用 是通过抑制炎症特异性COX-2酶的表达来介导的。我们的研究结果表明,C。obtusa精油是炎症特异性药物的新型来源。然而,需要进一步的研究来研究C. obtusa精油的具体作用机制和潜在的副作用。
致谢
该研究得到了由韩国政府资助的韩国国家研究基金会(NRF)资助(MEST;编号2010-0011433)。
缩写:
蚕蛾 | Chamaecyparis obtusa |
PGE2 | 前列腺素E 2 |
TNFα | 转化生长因子α |
COX-2 | 环氧合酶-2 |
NSAIDS | 非甾体类抗炎药 |
LPS | 内毒素脂多糖 |
参考
Simmons DL,Botting RM和Hla T:环氧合酶同工酶:前列腺素合成和抑制的生物学。Pharmacol Rev. 56:387-437。2004 浏览文章:谷歌学术:考研/ NCBI | |
Smith WL:营养必需脂肪酸和生物必不可少的环加氧酶。趋势Biochem Sci。33:27-37。2008. 查看文章:Google学术搜索:PubMed / NCBI | |
Koki A,Khan NK,Woerner BM,et al:Cyclooxygenase-2 in human pathological disease。Adv Exp Med Biol。507:177-184。2002. 查看文章:Google学术搜索:PubMed / NCBI | |
Zha S,Yegnasubramanian V,Nelson WG,Isaacs WB和De Marzo AM:癌症中的环氧合酶:进展和前景。巨蟹座Lett。215:1-20。2004 浏览文章:谷歌学术:考研/ NCBI | |
Cuzick J,Otto F,Baron JA等:用于癌症预防的阿司匹林和非甾体抗炎药:国际共识声明。柳叶刀Oncol。10:501-507。2009. 查看文章:Google学术搜索:PubMed / NCBI | |
Thun MJ和Blackard B:NSAIDs的药理作用以及对长期预防性使用阿司匹林预防癌症的风险和益处的影响。最新结果Cancer Res。181:215-221。2009. 查看文章:Google学术搜索:PubMed / NCBI | |
Mukherjee D:选择性环氧合酶-2(COX-2)抑制剂和心血管事件的潜在风险。Biochem Pharmacol。63:817-821。2002. 查看文章:Google学术搜索:PubMed / NCBI | |
Coruzzi G,Venturi N和Spaggiari S:新型非甾体类抗炎药的胃肠安全性:选择性COX-2抑制剂及其他。Acta Biomed。78:96-110。2007. PubMed / NCBI | |
Li W,Zhou P,Zhang Y和He L: 鱼腥草(Houttuynia cordata),一种具有抗炎活性的新型选择性COX-2抑制剂。J Ethnopharmacol。133:922-927。2011. 查看文章:Google学术搜索 | |
Joo SS,Yoo YM,Ko SH,等:来自Chamaecypris obtusa的精油对特应性皮炎样皮肤病变的发展和Th细胞因子的抑制的影响。J Dermatol Sci。60:122-125。2010. PubMed / NCBI | |
Singh BK,Tripathi M,Chaudhari BP,Pandey PK和Kakkar P:天然萜烯可防止线粒体功能障碍,氧化应激和大鼠尼美舒利 - 肝毒性过程中凋亡蛋白的释放。PLoS One。7:e342002012。 查看文章:Google学术搜索 | |
Lee GS,Byun HS,Kim MH,et al:Acer mono在具有低钙饮食诱导的骨质疏松症样症状的动物中的有益效果。Br J Nutr。100:1011-1018。2008. 查看文章:Google学术搜索:PubMed / NCBI | |
Lee GS,Hong EJ,Gwak KS,等:Chamaecyparis obtusa的精油通过诱导血管内皮生长因子基因促进毛发生长。Fitoterapia。81:17-24。2010。 | |
Lee SC,Ju SA,Pack HN等:4-1BB(CD137)是快速清除单核细胞增生李斯特氏菌感染所必需的。感染免疫。73:5144-5151。2005. 查看文章:Google学术搜索:PubMed / NCBI | |
Hasegawa H,Suzuki K,Nakaji S和Sugawara K:使用光泽精和鲁米诺依赖的化学发光分析和评估嗜中性粒细胞以96孔微孔板形式产生活性氧的能力。J Immunol方法。210:1-10。1997. 查看文章:Google学术搜索 | |
Vo TT,An BS,Yang H,Jung EM,Hwang I和Jeung EB:Calbindin-D9k作为敏感的分子生物标志物,用于评估雌激素化学物质对GH3大鼠垂体细胞的协同作用。Int J Mol Med。30:1233至40年。2012. PubMed / NCBI | |
Takayama C,de-Faria FM,de Almeida AC,et al:来自Hyptis spicigera Lam 的精油的胃保护和溃疡愈合作用。(唇形科)。J Ethnopharmacol。135:147-155。2011. 查看文章:Google学术搜索:PubMed / NCBI | |
Edris AE:精油及其各种挥发性成分的药物和治疗潜力:综述。Phytother Res。21:308-323。2007 浏览文章:谷歌学术:考研/ NCBI | |
Silva FV,GuimarãesAG,Silva ER等:香芹酚的抗炎和抗溃疡活性,香芹酚是一种存在于牛至精油中的单萜。J Med Food。15:984-991。2012. 查看文章:Google学术搜索:PubMed / NCBI | |
Lima DK,Ballico LJ,Rocha Lapa F,et al:来自Piper aleyreanum C.DC的啮齿动物精油的抗伤害性,抗炎和胃抗溃疡活性的评价。J Ethnopharmacol。142:274-282。2012. 查看文章:Google学术搜索:PubMed / NCBI | |
Veras HN,Araruna MK,Costa JG,等:Lippia sidoides Cham 精油的局部抗炎活性:可能的作用机制。Phytother Res。27:179-185。2012. 查看文章:Google学术搜索 | |
Hennebert O,Pelissier MA,Le Mee S,WülfertE和Morfin R:7β-羟基 - 表雄酮诱导的结肠炎大鼠的抗炎作用和前列腺素模式的变化。J Steroid Biochem Mol Biol。110:255-262。2008. 查看文章:Google学术搜索:PubMed / NCBI | |
Raymundo LJ,Guilhon CC,Alviano DS,等:Hyptis pectinata(L.)Poit精油的抗炎和抗伤害感受活性的表征。J Ethnopharmacol。134:725-732。2011. 查看文章:Google学术搜索:PubMed / NCBI | |
Yoon WJ,Kim SS,Oh TH,Lee NH和Hyun CG:Cryptomeria japonica精油抑制耐药性皮肤病原体和LPS诱导的一氧化氮和促炎细胞因子产生。Pol J Microbiol。58:61-68。2009年。 | |
Chao LK,Hua KF,Hsu HY等:肉桂醛通过抑制细胞内信号传导抑制单核细胞/巨噬细胞分泌促炎细胞因子。食品化学毒性。46:220-231。2008. 查看文章:Google学术搜索:PubMed / NCBI | |
Takeda K,Kaisho T和Akira S:Toll样受体。Annu Rev Immunol。21:335-376。2003. 查看文章:Google学术搜索 | |
Katsukawa M,Nakata R,Takizawa Y,Hori K,Takahashi S和Inoue H:Citral是柠檬草油的一种成分,可激活PPARα和γ并抑制COX-2的表达。Biochim Biophys Acta。1801:1214-1220。2010. 查看文章:Google学术搜索:PubMed / NCBI |
原文
Abstract
Essential oils are concentrated hydrophobic liquids containing volatile aromatic compounds from plants. In the present study, the essential oil of Chamaecyparis obtusa (C. obtusa), which is commercially used in soap, toothpaste and cosmetics, was extracted. Essential oil extracted from C. obtusa contains several types of terpenes, which have been shown to have anti-oxidative and anti-inflammatory effects. In the present study, we examined the anti-inflammatory effects of C. obtusa essential oil in vivo and in vitro following the induction of inflammation by lipopolysaccharides (LPS) in rats. While LPS induced an inflammatory response through the production of prostaglandin E2 (PGE2) in the blood and peripheral blood mononuclear cells (PMNCs), these levels were reduced when essential oil was pre-administered. Additionally, the mechanism of action underlying the anti-inflammatory effects of C. obtusa essential oil was investigated by measuring the mRNA expression of inflammation‑associated genes. LPS treatment significantly induced the expression of transforming growth factor α (TNFα) and cyclooxygenase-2 (COX-2) in rats, while C. obtusa essential oil inhibited this effect. Taken together, our results demonstrate that C. obtusa essential oil exerts anti‑inflammatory effects by regulating the production of PGE2 and TNFα gene expression through the COX-2 pathway. These findings suggest that C. obtusa essential oil may constitute a novel source of anti-inflammatory drugs.
Introduction
Inflammation is the most common cause of clinical pain resulting from tissue injury. The primary function of pain is to protect the organism from potential tissue-damaging stimuli. The aim of drug treatment for inflammation is to normalize pain sensitivity; however, drugs that are currently available are associated with critical side-effects and low efficacy. Non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to be effective in the treatment of various disorders with inflammation and have fewer side-effects compared with steroids (1). NSAIDs function through binding to the cyclooxygenase (COX) enzymes to inhibit the production of prostaglandins from arachidonic acid. Two isoforms of the COX protein, COX-1 and -2, exist; COX-1 is constitutively expressed in the majority of organs and cells, whereas COX-2 is generally absent and is induced upon stimulation by inflammatory stimuli, including endotoxin lipopolysaccharides (LPS). This suggests that COX-2 is an inflammation-specific isoform (1–3). NSAIDs and COX-2 selective inhibitors are currently used to treat several inflammatory disorders (4–6). However, selective inhibitors of COX-2 have been associated with a risk of myocardial infarction and stroke. The recent withdrawal of conventional NSAIDs and selective COX-2 inhibitors due to their adverse cardiovascular and gastric side-effects has led to the development of alternative anti-inflammatory agents (7–9). Therefore, plant-derived natural agents, such as essential oil, are considered to be useful sources for the next generation of anti-inflammatory drugs.
Essential oils are concentrated hydrophobic liquids containing volatile aromatic compounds from plants. They are extracted by distillation, expression or solvent extracts, and are used in perfumes, cosmetics, soaps and other products, for adding scents to incense and household products and for flavoring food and drink. Chamaecyparis obtusa (C. obtusa), a tropical tree species found in Japan and the southern region of South Korea, is used for construction and furniture due to its advantage in structural properties and natural scent. Its essential oil is extracted from pruned leaves and twigs of the C. obtusa tree, and has been commercially used in soap, toothpaste and cosmetics as a functional additive. The C. obtusa essential oil contains several types of terpenes that have been shown to exert anti-oxidative and anti-inflammatory effects, including sabinene, limonene, bornyl acetate, borneol, α-terpineol and elemol, while essential oils from fruits contain myrcene, γ-terpinene, p-cymene, borneol, α-terpineol and β-caryophyllene (10,11).
In the present study, the anti-inflammatory property of the essential oil from C. obtusawas investigated in vivo and in vitro, and the ability of C. obtusa to serve as a source of novel pharmaceuticals was determined.
Materials and methods
Animals and treatment
Male Sprague-Dawley (SD) rats (9 weeks old) were obtained from Koatech Co., Ltd. (Pyeongtaek-si, Korea). All the animals were housed in polycarbonate cages and acclimated in an environmentally controlled room (23±2°C; relative humidity, 50±10%; frequent ventilation; and a 12-h light/dark cycle) prior to use as previously described (12). C. obtusa essential oil used in this study was provided by Enbita Co., Ltd. (Seoul, Korea) and was produced through steam distillation of pruned twigs and leaves of the C. obtusa tree, according to previously described methods (13). The rats were treated with C. obtusa essential oil to investigate its effects. A total of 50 ml tap water or essential oil solution (25, 50 or 100%) was administered to the rats for 2 weeks. Rats in the control group were treated with a saline solution (0.9% NaCl), and the remaining rats (treatment group) were treated with 0, 25, 50 or 100% solutions of C. obtusa essential oil in the presence or absence of LPS (Sigma-Aldrich, St. Louis, MO, USA). Rats in the LPS-treated group (LPS-positive group) were administered 1 μg/kg LPS via an intraperitoneal (i.p.) injection 8 h prior to sacrifice; rats in the control group for LPS (LPS-negative group) were injected with an equivalent volume of saline solution at the same time-point with LPS. All the experimental procedures were approved by the Ethics Committee of the Chungbuk National University (Cheongju, Korea).
Peripheral blood mononuclear cell (PMNC) isolation
PMNCs were isolated from rat peripheral blood vessels using a double density gradient centrifugation method as previously described (14,15). Briefly, heparinized blood samples were overlaid on Histopaque-1077 solution (specific gravity, 1.077; Sigma-Aldrich) and Histopaque-1119 solution (specific gravity, 1.199; Sigma-Aldrich), centrifuged for 40 min at 700 × g and washed three times with cold phosphate-buffered saline (PBS). The viability of PMNCs, determined by trypan blue dye exclusion, was >97%. The PMNCs were resuspended in RPMI-1640 medium (Sigma-Aldrich) supplemented with 2 mM L-glutamine, 0.02 mg/ml gentamicin and 5% heat-inactivated fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA) for use in subsequent experiments.
Prostaglandin E2 (PGE2) ELISA
For the in vivo experiment, rats were treated with various solutions of C. obtusaessential oil in the presence or absence of LPS. Following treatment, serum was collected from peripheral blood vessels and subjected to a PGE2 ELISA assay. For the in vitro assay, rats were treated with the essential oil solutions for 2 weeks. PMNCs were harvested from rat peripheral blood vessels and plated on 24-well plates at a density of 0.5×105. The PMNCs were then stimulated with 10 ng/ml LPS for the indicated times, and the supernatant was collected. For the PGE2 ELISA assay, the collected serum and supernatant were analyzed using a monoclonal PGE2 ELISA kit (Cayman Chemical, Ann Arbor, MI, USA).
Quantification of mRNA by RT-PCR
Total cellular RNA was extracted using TRIzol reagent (Invitrogen), and the concentration of RNA was determined at 260 nm. RT-PCR was performed as previously described (13). Briefly, total RNA (1 μg) was reverse transcribed into complementary DNA (cDNA) using M-MLV reverse transcriptase (Invitrogen) and a random primer (9-mer; Takara Bio, Inc., Shiga, Japan). The cDNA (1 μl) was used for PCR under standard conditions (13,16); denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for 1 min. The primer sequences used were as follows: COX-2 sense, 5′-TACCCGGACTGGATTCTACG-3′ and antisense, 5′-AAGTTGGTGGGCTGTCAATC-3′; TNFα sense, 5′-CTGAGCTCAAGCCCTGGTAT-3′ and antisense, 5′-GGTCAGAGTAATGGGGGTCA-3′; and cytochrome c oxidase subunit 1 (1A) sense, 5′-CCAGGGTTTGGAATTATTTC-3′ and antisense, 5′-GAAGATAAACC CTAAGGCTC-3′, which was used as an internal control. The PCR products (10 μl) were separated on 2% agarose gel and stained with ethidium bromide (EtBr). The gel image was captured and analyzed using Quantity One software (Gel Doc EQ; Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were analyzed by non-parametric one-way analysis of variance using the Kruskal-Wallis test, followed by Dunnett’s test for multiple comparisons. Data values were converted to ranks for these tests. All the statistical analyses were performed with SPSS for Windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of essential oil from C. obtusa on serum PGE2 concentration
The regulation of serum PGE2 by essential oils from C. obtusa was investigated. Rats were treated with various concentrations of the essential oil for 2 weeks and 1 μg/kg LPS was injected to induce an inflammatory reaction following treatment with the essential oil.
To analyze the levels of serum PGE2, ELISA with a specific antibody for PGE2 was performed. The levels of LPS-induced serum PGE2 were significantly reduced in animals treated with essential oil at a dose of 25 mg/kg (Fig. 1). Other concentrations ofC. obtusa essential oil (50 and 100 mg/kg) also reduced the levels of serum PGE2; however, these results were not statistically significant. Next, the effects of essential oil were evaluated, using PMNCs to verify the increase in serum PGE2 in the rats. PMNCs were isolated following treatment with various concentrations of the essential oil for 2 weeks and administration of 10 ng/ml LPS for 12 (Fig. 2A) or 24 h (Fig. 2B). The essential oil was shown to have protective effects on LPS-induced inflammation by reducing PGE2 secretion in the PMNCs. The 12-h LPS-induced PGE2 secretion level was inhibited by treatment with the essential oil at all the doses examined, and the maximum inhibition was observed at a dose of 50 mg/kg. The inhibition of PGE2production induced by LPS treatment for 24 h was shown to be significant only when 100 mg/kg of the essential oil was applied.
 | Figure 1Effects of various doses of Chamaecyparis obtusa essential oil on lipopolysaccharide (LPS)-induced concentrations of prostaglandin E2 (PGE2) in the serum of rats. The rats were treated via oral administration of the essential oils for 2 weeks, and 1 μg/kg LPS was intraperitoneally injected on the last day of treatment. Serum samples were collected from rat blood samples 8 h after the LPS injection. The serum samples were filtered with a 0.45-μm pore size membrane and PGE2 concentrations were determined using ELISA. *P=0.036 compared with control group, as determined by one-way ANOVA followed by a Dunnett’s test (n=4). |
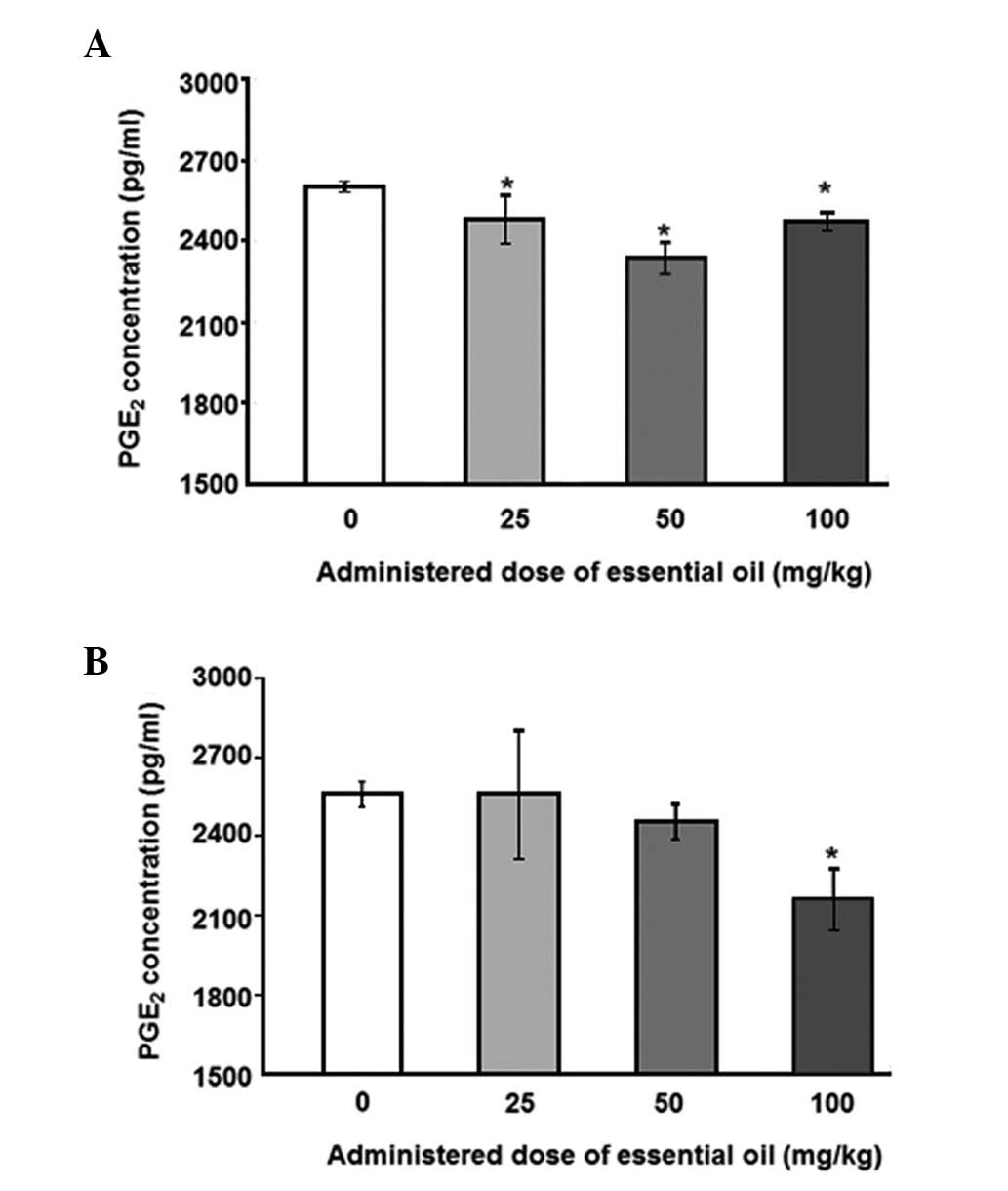 | Figure 2Effect of various doses of Chamaecyparis obtusa essential oil on lipopolysaccharide (LPS)-induced production of prostaglandin E2 (PGE2) using peripheral blood mononuclear cells (PMNCs). PMNCs were isolated from blood samples obtained from rats treated via oral administration of the essential oil for 2 weeks. Isolated PMNCs were stimulated by 10 ng/ml LPS for (A) 12 or (B) 24 h, and the culture supernatants were harvested and filtered with a 0.45-μm pore size membrane. PGE2 concentrations in the culture supernatant were determined using ELISA. *P≤0.001 compared with control group, as determined by one-way ANOVA followed by a Dunnett’s test (n=4). |
Effect of essential oils from C. obtusa on expression of the inflammation-related genes TNFα and COX-2 in lung tissue
To examine the possible mechanisms of action underlying the anti-inflammatory effects of the essential oil, we analyzed the expression of inflammation-associated genes in the lungs of rats following treatment with the essential oil in the presence or absence of LPS. As expected, LPS enhanced the expression of TNFα (Fig. 3). LPS-induced TNFα expression in the lung was reduced by administration of the essential oil in a dose-dependent manner, where the most potent effect was observed at a dose of 100 mg/kg (56% reduction).
 | Figure 3Effect of Chamaecyparis obtusa essential oil (EO) on lipopolysaccharide (LPS)-induced expression of tumor necrosis factor α (TNFα) mRNA. The rats were treated via oral administration of various doses of the essential oil for 2 weeks, and 1 μg/kg LPS was intraperitoneally injected on the last day of treatment. The mRNA samples were extracted from the lungs of rats 8 h after the LPS injection and analyzed by RT-PCR with primers for TNFα as described in Material and methods. aP<0.05 compared with the LPS-negative group; bP<0.05 compared with the LPS-positive control group. |
COX-2 mRNA expression was also assessed to determine the mechanism via which the essential oil exerts it anti-inflammatory effects (Fig. 4). The expression of COX-2 was increased by LPS treatment (up to 5-fold) compared with the control group (Fig. 4). The induction of COX-2 expression by LPS treatment was inhibited by pre-administration of the essential oil; this inhibition was most marked at a dose of 50 mg/kg.
 | Figure 4Effect of Chamaecyparis obtusa essential oil (EO) on lipopolysaccharide (LPS)-induced expression of cyclooxygenase-2 (COX-2) mRNA. The rats were treated via oral administration of various doses of the essential oil for 2 weeks, and 1 μg/kg LPS was intraperitoneally injected on the last day of treatment. The mRNA samples were extracted from the lungs of rats 8 h after the LPS injection, and COX-2 expression was analyzed by RT-PCR. aP<0.05 compared with the LPS-negative group; bP<0.05 compared with the LPS-positive control group. |
Discussion
Since ancient times, plants have been used for their medicinal properties, which are partially attributed to essential oils (17). Essential oils are natural, complex and multi-component oils composed mainly of terpenes with additional components (18). Essential oils are widely used to prevent and treat human diseases, including cancer and cardiovascular diseases. Furthermore, their bioactivity as antibacterial, antiviral, antioxidant and antidiabetic agents has previously been reported (18). Recent studies have demonstrated the anti-inflammatory properties of essential oils from several plants (19–21). In the present study, we demonstrated that the essential oil from C. obtusa has an anti-inflammatory effect on LPS-induced inflammation in rats. The serum concentration of PGE2 was reduced when the rats were treated with the essential oil. In addition, the synthesis of PGE2 from blood PMNCs showed concomitant results with that of serum. These results suggest that PGE2 is synthesized by blood monocytes following stimulation by LPS, and that this inflammatory process is ameliorated by C. obtusa essential oil.
PGE2 is produced in the body and accumulates with LPS treatment through stimulation of the COX pathway. The quantitative analysis of PGE2 represents inflammatory activities in vivo and in vitro(22). In the present study, LPS-induced PGE2 production was reduced in the serum and PMNCs of rats by treatment with C. obtusa essential oil. Similar results have been previously reported; Hyptis pectinata essential oil has been shown to have anti-inflammatory effects by inhibiting the production of PGE2 and nitric oxide (23). Cryptomeria japonica essential oil has been demonstrated to inhibit the LPS-induced production of PGE2 and cytokines in macrophages, including interleukin (IL)-1β and IL-6 (24); furthermore, the chemical composition of Cryptomeria japonica essential oil was analyzed and its major components were revealed to be elemol and sabinene. Elemol and sabinene are also known to be terpenes of C. obtusa essential oil (10,11).
In the present study, we investigated the effects of C. obtusa essential oil on the regulation of TNFα mRNA expression following LPS treatment. As expected, the expression of TNFα was increased in the lung tissues of rats following LPS treatment, which implied induction of the immune response by LPS. Furthermore, the induced expression of TNFα was abrogated by treatment with C. obtusa essential oil. To verify the mechanism of action underlying the anti-inflammatory effects of C. obtusaessential oil, we also examined the regulation of COX-2 expression levels. Pathogen-associated immune responses are antigenic, and are able to activate monocytes or macrophages to synthesize various inflammatory cytokines, including IL-1, IL-6 and TNF, which have been reported to be mediated via COX expression (24–26). The expression of COX-2 was increased following treatment with LPS, and this upregulation was inhibited by C. obtusa essential oil in a dose-dependent manner. These results suggest that the anti-inflammatory effects of this essential oil occur via COX-2-mediated regulation of PGE2 and TNFα signaling pathways.
The anti-inflammatory effects of essential oils through the COX-2 signaling pathway have been previously demonstrated; the essential oil from Houttuynia cordata has been shown to possess anti-inflammatory properties through inhibiting the LPS-induced production of PGE2(9). In this study, the anti-inflammatory effects of the essential oil were mediated by the inhibition of COX-2 gene expression, while COX-1, the non-inflammation-specific isoform, was not involved. Katsuawa et al(27) also demonstrated that the essential oil of lemongrass suppresses COX-2 promoter activity in human macrophage-like U937 cells.
In conclusion, our results demonstrate that C. obtusa essential oil exerts it anti-inflammatory effects by regulating the production of PGE2 in the blood and the gene expression of TNFα in rats. The anti-inflammatory effects of C. obtusa essential oil are mediated by inhibiting the expression of the inflammation-specific COX-2 enzyme. Our results suggest that C. obtusa essential oil is a novel source of inflammation-specific pharmacological drugs. However, further studies are required to investigate the specific mechanisms of action and potential side-effects of C. obtusa essential oil.
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST; no. 2010-0011433).
Abbreviations:
C. obtusa | Chamaecyparis obtusa |
PGE2 | prostaglandin E2 |
TNFα | transforming growth factor α |
COX-2 | cyclooxygenase-2 |
NSAIDS | nonsteroidal anti-inflammatory drugs |
LPS | endotoxin lipopolysaccharides |
References
Simmons DL, Botting RM and Hla T: Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 56:387–437. 2004. View Article : Google Scholar : PubMed/NCBI | |
Smith WL: Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem Sci. 33:27–37. 2008. View Article : Google Scholar : PubMed/NCBI | |
Koki A, Khan NK, Woerner BM, et al: Cyclooxygenase-2 in human pathological disease. Adv Exp Med Biol. 507:177–184. 2002. View Article : Google Scholar : PubMed/NCBI | |
Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB and De Marzo AM: Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 215:1–20. 2004. View Article : Google Scholar : PubMed/NCBI | |
Cuzick J, Otto F, Baron JA, et al: Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 10:501–507. 2009. View Article : Google Scholar : PubMed/NCBI | |
Thun MJ and Blackard B: Pharmacologic effects of NSAIDs and implications for the risks and benefits of long-term prophylactic use of aspirin to prevent cancer. Recent Results Cancer Res. 181:215–221. 2009. View Article : Google Scholar : PubMed/NCBI | |
Mukherjee D: Selective cyclooxygenase-2 (COX-2) inhibitors and potential risk of cardiovascular events. Biochem Pharmacol. 63:817–821. 2002. View Article : Google Scholar : PubMed/NCBI | |
Coruzzi G, Venturi N and Spaggiari S: Gastrointestinal safety of novel nonsteroidal antiinflammatory drugs: selective COX-2 inhibitors and beyond. Acta Biomed. 78:96–110. 2007.PubMed/NCBI | |
Li W, Zhou P, Zhang Y and He L: Houttuynia cordata, a novel and selective COX-2 inhibitor with anti-inflammatory activity. J Ethnopharmacol. 133:922–927. 2011. View Article : Google Scholar | |
Joo SS, Yoo YM, Ko SH, et al: Effects of essential oil from Chamaecypris obtusaon the development of atopic dermatitis-like skin lesions and the suppression of Th cytokines. J Dermatol Sci. 60:122–125. 2010.PubMed/NCBI | |
Singh BK, Tripathi M, Chaudhari BP, Pandey PK and Kakkar P: Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS One. 7:e342002012.View Article : Google Scholar | |
Lee GS, Byun HS, Kim MH, et al: The beneficial effect of the sap of Acer mono in an animal with low-calcium diet-induced osteoporosis-like symptoms. Br J Nutr. 100:1011–1018. 2008. View Article : Google Scholar : PubMed/NCBI | |
Lee GS, Hong EJ, Gwak KS, et al: The essential oils of Chamaecyparis obtusapromote hair growth through the induction of vascular endothelial growth factor gene. Fitoterapia. 81:17–24. 2010. | |
Lee SC, Ju SA, Pack HN, et al: 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect Immun. 73:5144–5151. 2005. View Article : Google Scholar : PubMed/NCBI | |
Hasegawa H, Suzuki K, Nakaji S and Sugawara K: Analysis and assessment of the capacity of neutrophils to produce reactive oxygen species in a 96-well microplate format using lucigenin- and luminol-dependent chemiluminescence. J Immunol Methods. 210:1–10. 1997. View Article : Google Scholar | |
Vo TT, An BS, Yang H, Jung EM, Hwang I and Jeung EB: Calbindin-D9k as a sensitive molecular biomarker for evaluating the synergistic impact of estrogenic chemicals on GH3 rat pituitary cells. Int J Mol Med. 30:1233–1240. 2012.PubMed/NCBI | |
Takayama C, de-Faria FM, de Almeida AC, et al: Gastroprotective and ulcer healing effects of essential oil from Hyptis spicigera Lam. (Lamiaceae). J Ethnopharmacol. 135:147–155. 2011. View Article : Google Scholar : PubMed/NCBI | |
Edris AE: Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 21:308–323. 2007. View Article : Google Scholar : PubMed/NCBI | |
Silva FV, Guimarães AG, Silva ER, et al: Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J Med Food. 15:984–991. 2012. View Article : Google Scholar : PubMed/NCBI | |
Lima DK, Ballico LJ, Rocha Lapa F, et al: Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C.DC in rodents. J Ethnopharmacol. 142:274–282. 2012. View Article: Google Scholar : PubMed/NCBI | |
Veras HN, Araruna MK, Costa JG, et al: Topical antiinflammatory activity of essential oil of Lippia sidoides Cham: possible mechanism of action. Phytother Res. 27:179–185. 2012. View Article : Google Scholar | |
Hennebert O, Pelissier MA, Le Mee S, Wülfert E and Morfin R: Anti-inflammatory effects and changes in prostaglandin patterns induced by 7beta-hydroxy-epiandrosterone in rats with colitis. J Steroid Biochem Mol Biol. 110:255–262. 2008. View Article : Google Scholar : PubMed/NCBI | |
Raymundo LJ, Guilhon CC, Alviano DS, et al: Characterisation of the anti-inflammatory and antinociceptive activities of the Hyptis pectinata (L.) Poit essential oil. J Ethnopharmacol. 134:725–732. 2011. View Article : Google Scholar : PubMed/NCBI | |
Yoon WJ, Kim SS, Oh TH, Lee NH and Hyun CG: Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Pol J Microbiol. 58:61–68. 2009. | |
Chao LK, Hua KF, Hsu HY, et al: Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 46:220–231. 2008. View Article : Google Scholar : PubMed/NCBI | |
Takeda K, Kaisho T and Akira S: Toll-like receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar | |
Katsukawa M, Nakata R, Takizawa Y, Hori K, Takahashi S and Inoue H: Citral, a component of lemongrass oil, activates PPARalpha and gamma and suppresses COX-2 expression. Biochim Biophys Acta. 1801:1214–1220. 2010. View Article : Google Scholar : PubMed/NCBI |






